“Dynamiclear ORO is a single application topical treatment for cold sores & fever blisters caused by herpes viruses. It has been scientifically formulated using an FDA approved Ingredient, Camphor, a pain reliever and a Patented Formula of natural ingredients that has proven Clinical efficacy against Herpes Simplex Viruses (HSV1 and HSV11). Dynamiclear ORO works by eliminating the virus on direct contact. It requires just one application per outbreak. The Patented Formula has been Clinical Proven to provide rapid reduction in symptoms of cold sores within 24-48 hours (in typical cases *) in just a single application. Dynamiclear ORO provides quick pain relief, dries up blisters & weeping sores and can be applied at any stage of an outbreak. (*In some cases, cold sores may take up to 72hrs or longer to clear up and may require more than one application, as shown in the Clinical Study.

Unique Easy to Use Procedure Pack
Directions to Use – How to Apply
Dynamiclear is applied topically to each Cold Sore Outbreak in a single application using the procedure pack supplied as per the illustrations below).
The procedure pack contains a cotton tip applicator and a glass vial containing the medicine in gel form. The cotton tip applicator must be inserted into the Vial and through capillary action the liquid is drawn to the top exposed end. It may be necessary to move the cotton tip applicator up and down to allow more medicine to flow. In few cases the product may not flow through, in that case reverse insert the cotton tip applicator into the glass vial. Always use the exposed end only when it is fully saturated with the medicine. Apply by holding the glass vial and pressing the exposed end of the cotton tip applicator gently onto the lesions for up to 30 seconds or more as needed. One thorough application (using all the medicine in the vial) must be applied to each outbreak. In case of an open wound such as a blister stage, a sharp sting may be felt. If symptoms persists after 24 hours apply another single application.
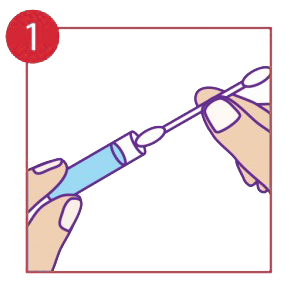
Insert cotton tip into the Dynamiclear vial.
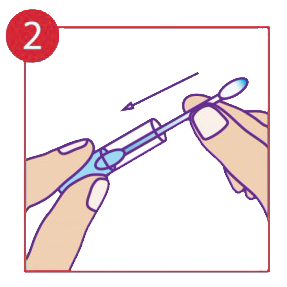
Push cotton tip into the liquid.
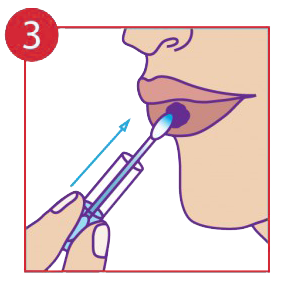
Place the cotton tip on the infected area and slide up the vial.
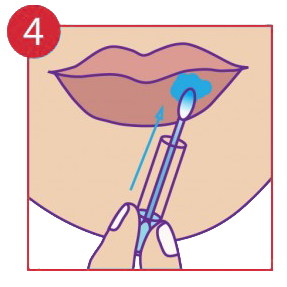
Apply pressure to the infected area thoroughly for 30 seconds or more.
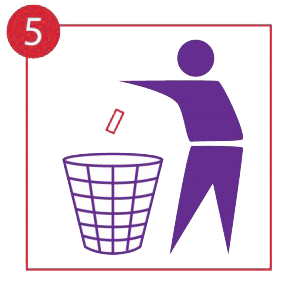
Dispose of hygienically.
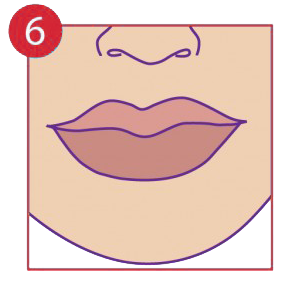
Keep dry and allow time to heal without disturbance.
Contra-Indications
Allergic reactions to Dynamiclear are known to occur. If you experience any adverse reaction discontinue the use (signs and symptoms listed below).
Wash affected area with copious amount of water and seek medical attention if required.
- Severe burning sensation or inflammation of lips.
- Severe inflammation of Lesions or blisters and it starts to weep or bleed
- Severe increases in pain over long period more than an hour or two. (Note: When Dynamiclear is applied to active cold sores you may experience an imminent sharp pain that indicates the medicine is working that should subside quickly. It is not necessarily an adverse reaction)
- Increased and excessive dryness of lips and mouth area if or when applying inside the lips (the mucosa membrane). Note: Dynamiclear should not be used inside the mouth area. Do not confuse Canker Sores with Cold Sores. Canker Sores are ulcers that appear mostly inside the mouth and the mucosa membrane inside the lips. Cold Sores mainly appears on or outside the lips
Who, When and Where not to use Dynamiclear:
- People who are allergic to Dynamiclear. First time uses if you have hypersensitive skin or are unsure perform a patch test by applying Dynamiclear lightly under your arm (a sensitive part of your skin, wait up to 3 to 5mins, if no severe burning sensation, inflammation or pain is experienced, use as directed)
- If pregnant seek medical advice from your Doctor or pharmacist before using Dynamiclear. Do not use during the first Trimester
- Do not use in your eyes or mouth
- When your doctor advises not to use Dynamiclear
- If you are allergic to any of the ingredients in the product.
- Children under the age of 12 should seek medical advice prior to using Dynamiclear
- People who are immunocompromised should seek medical advice prior to using Dynamiclear.
- People who are not sure should seek medical advice from a Pharmacist or Doctor prior to using Dynamiclear.
Dynamiclear is Gold Standard in Cold Sore treatment
| Dynamiclear | |
| Pain Reliever (Analgesic) | ✓ |
| Antiviral | ✓ |
| Viricidal (Inactivates The Virus) | ✓ |
| Anti-inflammatory | ✓ |
| Anti-fungal | ✓ |
| Anti-bacterial | ✓ |
| Wound Healing | ✓ |
| Stops Transmissions | ✓ |
| Reduces Healing Time | ✓ |
| Number of Daily Applications | Once |
| When to Apply | At Any Stage |
Drug Facts
NDC Code(s): 49836-029-02
Packager: RX PHARMA-PACK, INC.
Category: HUMAN OTC DRUG LABEL
DEA Schedule: None
Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
DRUG LABEL INFORMATION
If you are a consumer or patient please visit this version.
VIEW ALL SECTIONS
ACTIVE INGREDIENT
Camphor 3%
PURPOSE
Fever blister/ cold sore treatment/ pain reliever (external analgesic)
USES
Pain relief – Relieves burning and itching – Fever blister/cold sore relief
WARNINGS
For external use only.
Ask a doctor or pharmacist before use if you have any questions about this product.
When using this product do not get into eyes.
Stop use and ask a doctor if condition worsens symptoms last more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
DIRECTIONS
| Adults and children 12 years and over | Single application |
| Children 2 to under 12 years | use under adult supervision only |
| Children under 2 years | Consult a doctor |
Apply a single application to each outbreak as needed. Repeat application may not be necessary. If symptoms persist over 24-hours, apply another single application.
OTHER INFORMATION:
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
STORAGE CONDITIONS
Store between 10°-25°C (50°F-77°F)
Avoid heat and direct sunlight
For the procedure pack to work properly keep the product between 10°C to 25°C). If the temperature of the product drops below 5°C the medicine may become very thick. In this case take the vial and rub between your palm of your hands until temperature increase. You will notice the cottontip applicator sliding into the product more easily. In case the medicine is not flowing through with ease, reverse insert the cotton tip applicator. Ensure one end of the applicator is fully saturated and the other end is immersed into the medicine in the glass vial)
INACTIVE INGREDIENTS
Aloe vera, calendula officinalis, cupric sulfate, glycerol, hypericum perforatum, isopropyl alcohol, polyethylene glycol, polysorbate, vitamin E
QUESTIONS OR COMMENTS?
Rx Pharma Pack 1-844-632-7898
PRINCIPAL DISPLAY PANEL
For External Use Only
TWIN PACK
(2x single application packs)
NDC# 49836-029-02
ORO – Dynamiclear™
Camphor 3%
✓ Pain Relief ✓ Relieves Burning
✓ Single Application ✓ Relieves Itching
(Amount: 2x (0.75 mL Each) Single Application Swabs Per Unit Pack)
ALWAYS READ INSTRUCTIONS BEFORE USE.
- Carefully remove cap and slowly insert cotton tip into glass vial.
- Gently push cotton tip into the liquid. Hold onto vial. Do not remove cotton tip from vial.
- Apply to infected area with a gentle pressure for 30 seconds then discard. Keep area dry.
Exclusive US Distributor: Rx Pharma Pack
245 Oser Avenue, Hauppauge, NY 11788
FEVER BLISTER / COLD SORE TREATMENT
(EXTERNAL ANALGESIC )
INGREDIENTS AND APPEARANCE
Product Information
| ORO DYNAMICLEAR camphor liquid | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER – RX PHARMA-PACK, INC. (962149634) | |||||||||||||||||||||||||
| ESTABLISHMENT | |||
| Name | Address | ID/FEI | Business Operations |
| RX PHARMA-PACK, INC. | 962149634 | MANUFACTURE(49836-029) , PACK(49836-029) | |
What are cold sores?
Cold Sores are viral infections that appear as small, painful fluid filled blisters (vesicles) in and around the lips, nose mouth and chin, (Orofacial region. Hence Cold Sores are also called Orofacial Herpes or Herpes Labialis (meaning Herpes on Lips).
Stages of Cold Sores Outbreak

Day 1: Prodromal (Erythema) – A tingling, itching or burning sensation is experienced beneath the skin.
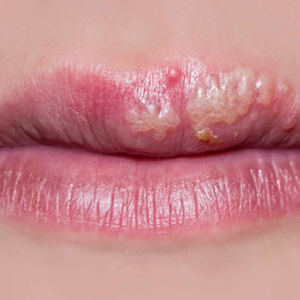
Day 2 to 3: Blistering (Vesiculation) – The emergence of painful, fluid filled vesicles occurs 24-48hrs after prodromal stage, and is the first sign of a cold-sore.
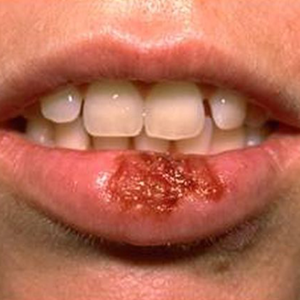
Day 4: Ulceration and Weeping – The most contagious and painful stage as rupturing of the vesicles occurs. This results in 1-3mm shallow, grey-white ulcers on erythemous bases of the skin.
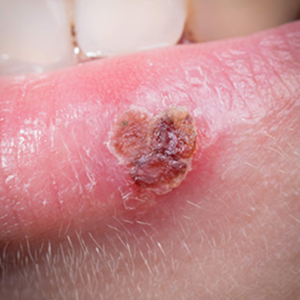
Day 5 to 8: Crusting Stage (Induration) – The blisters dry up, forming a yellow or brownish crust which eventually falls off. This scab will easily crack or break and care of the scab is important.
Day 9 to 14: Healing Stage (Recovery) – A series of scabs form on the lesion. They eventually flake as the cold-sore heals, usually without scarring.
Management of HSV Infections
- Antiviral Medication – Often the most effective way in managing the occurrence of outbreaks
- Topical Medicine – The most widely used treatments include acyclovir and derivatives. These treatments need to be applied multiple times a day at the very onset of cold sores (the very early prodromal stages) to suppress the virus. Studies show that there are now acyclovir resistant strains of HSV.
- Oral Medications – Treatments include acyclovir and derivatives as well as supplements such as lysine HCL.
- Preventative measures – These are essential to stop the spread of infection and include:
- Prevention – Healthy immune system and hygiene
- Wash hands thoroughly before and after applying medication
- Avoid physical contact with infected person – Practice safe sex
- Avoid sharing personal items as well as food and drink with infected person
- Novel Treatment – Dynamiclear has been clinically tested to provide quick and fast relief from symptoms of Cold Sores
Clinically Proven
Efficacy and Tolerability Assessment of a Topical Formulation Containing Copper Sulfate and Hypericum perforatum on Patients With Herpes Skin Lesions: A Comparative, Randomized Controlled Trial
Abstract
Background: Topical Acyclovir has moderate efficacy on recurrent HSV symptoms, requiring repeat applications for several days. Topical Dynamiclear, which requires only a single dose application, may provide a more effective and convenient treatment option for symptomatic management of HSV.
Objectives: The study assessed the comparative efficacy and tolerability of a single use, topical formulation containing copper sulfate pentahydrate and Hypericum perforatum that is marketed as Dynamiclear™ to a topical 5% Acyclovir cream standard preparation and use.
Methods: A prospective, randomized, multi-centered, comparative, open-label clinical study was conducted. A total of 149 participants between 18 and 55 years of age with active HSV-1 and HSV-2 lesions were recruited for the 14-day clinical trial. Participants were randomized into two groups: A (n=61), those receiving the Dynamiclear formulation, and B (n=59), those receiving 5% Acyclovir. Efficacy parameters were assessed via physical examination at baseline (day 1), day 2, 3, 8, and 14. Laboratory safety tests were conducted at baseline and on day 14.
Results: Use of the Dynamiclear formulation was found to have no significant adverse effects and was well tolerated by participants. All hematological and biochemical markers were within normal range for the Dynamiclear group. Statistically, odds for being affected by burning and stinging sensation were 1.9 times greater in the Acyclovir group in comparison to the Dynamiclear group. Similarly, the odds of being affected by symptoms of acute pain, erythema and vesiculation were 1.8, 2.4, and 4.4 times higher in the Acyclovir group in comparison to the Dynamiclear group.
Conclusions: The Dynamiclear formulation was well tolerated, and efficacy was demonstrated in a number of measured parameters, which are helpful in the symptomatic management of HSV-1 and HSV-2 lesions in adult patients. Remarkably, the effects seen from this product came from a single application.
J Drugs Dermatol. 2012;11(2):209-215.
Why use multiple applications to treat cold sores when a single application can inactivate the virus.
Try DynamiClear.
Buy Now